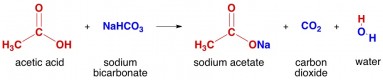
When this experiment is repeated with nitric or sulfuric acid instead of \(HCl\), it yields the same results: the clear limewater turns milky, indicating the production of carbon dioxide. Sulfuric acid, #"H"_2"SO"_4#, will react with sodium carbonate, #"Na"_2"CO"_3#, to form sodium sulfate, #"Na"_2"SO"_4#, and carbonic acid, #"H"_2"CO"_3#.

Log in here. xb```b``a`e``ec@ >+ Nla`0oh3i
BX*$=Ja{[#nU,qJ{y"AA2:L
Please contact your Account Manager if you have any query.

See all questions in Chemical Reactions and Equations. The reaction produces sodium carbonate, water, and carbon dioxide.

In the reaction, H2SO4 + Na2CO3 -> Na2SO4 + CO2 + H2O, bubbles of carbon dioxide gas (CO2) are produced. 2) Why is thiosulfate titration an iodometric procedure? 0000009259 00000 n
Like any chemical equation, a neutralization chemical equation must be properly balanced.

endstream
endobj
675 0 obj<>/OCGs[677 0 R]>>/PieceInfo<>>>/LastModified(D:20070109112353)/MarkInfo<>>>
endobj
677 0 obj<>/PageElement<>>>>>
endobj
678 0 obj<>/Font<>/ProcSet[/PDF/Text]/ExtGState<>>>/StructParents 0>>
endobj
679 0 obj<>
endobj
680 0 obj<>
endobj
681 0 obj<>
endobj
682 0 obj<>
endobj
683 0 obj<>
endobj
684 0 obj<>
endobj
685 0 obj<>stream
US toll free: 1-844 677 4151, General enquiries: info@sciencephoto.com Legal. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. 674 31
Latest answer posted October 04, 2014 at 6:07:29 PM. London 2022 eNotes.com, Inc. All Rights Reserved. 0000004224 00000 n
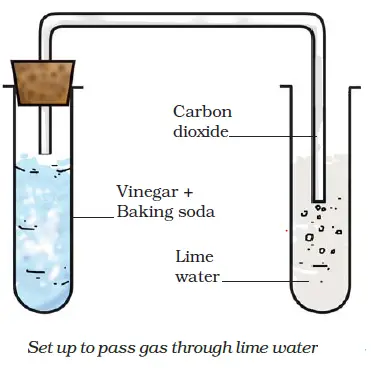
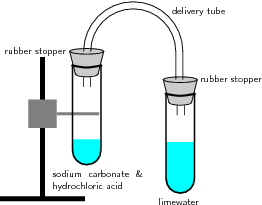
In this problem the reaction satisfies this rule, and sodium sulphate `Na_2 S O_4` and carbonic acid are formed. This is a double displacement reaction, so the cations and anions swap to create new products. Figure \(\PageIndex{1}\) demonstrates this type of reaction: In this reaction setup, lime water, a dilute calcium hydroxide (\(Ca(OH)_2\)) solution, is poured into one of the test tubes and sealed with a stopper. Please enable it in your browser. 0000006566 00000 n
Latest answer posted March 04, 2016 at 12:51:37 AM.

0000001437 00000 n
Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. This image is not available for purchase in your country. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky. What are the four basic functions of a computer system? Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license.


674 0 obj<>
endobj
0000003661 00000 n
0000005766 00000 n

Acids and bases react chemically with each other to form salts. Sodium Carbonate/Sulfuric Acid Reaction, 3 of 3. What is the full equation for nitric acid+potassium carbonate? As the reaction proceeds, the limewater on the turns from clear to milky; this is due to the \(CO_2(g)\) reacting with the aqueous calcium hydroxide to form calcium carbonate, which is only slightly soluble in water.
0000000933 00000 n
TURTLE ROCK SCIENTIFIC/SCIENCE PHOTO LIBRARY. \(\ce{2HCl(aq) + K2S \rightarrow H2S (g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2CO2 \rightarrow H2O (l) + CO2(g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2SO2 \rightarrow H2O (l) + SO2(g) + 2KCl (aq)}\), \(\ce{NH4Cl(aq) + KOH \rightarrow H2O (l) + NH3(g) + 2KCl (aq)}\). %%EOF
In the above redox reaction, neutral zinc is oxidized to \(Zn^{2+}\), and the acid, \(H^+\), is reduced to \(H_2(g)\). For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows: \[\ce{NaOH (aq) + HCl (aq) \rightarrow NaCl (aq) + H_2O ()} \label{Eq2} \].
Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. 676 0 obj<>stream
Some features of this website require JavaScript. So the (potentially unbalanced) reaction is, `H_2 S O_4 +Na_2 C O_3 = Na_2 S O_4 +H_2 O +C O_2.`.
Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team. Hydrocyanic acid (\(\ce{HCN(aq)}\)) can be neutralized by potassium hydroxide (\(\ce{KOH(aq)}\)).


Sodium carbonate reacts with sulfuric acid, 3 of 3.

endstream
endobj
704 0 obj<>/W[1 1 1]/Type/XRef/Index[31 643]>>stream
0000002618 00000 n
0000019047 00000 n
startxref
around the world. The oxidation of metals by strong acids is another common example of a gas evolution reaction.

Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). 0000008843 00000 n
\[\ce{KOH (aq) + HCN(aq) KCN (aq) + H2O()} \nonumber \].
Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Also carbonic acid is unstable and forms water `H_2 O` and carbon dioxide `C O_2` which goes out as a gas. with coefficients all understood to be one.
The reaction of acid and base to make water and a salt is called neutralization. Write and balance the equation for the reaction of hydrochloric acid (H2SO4) and sodium hydroxide to produce sodium sulfate and water.
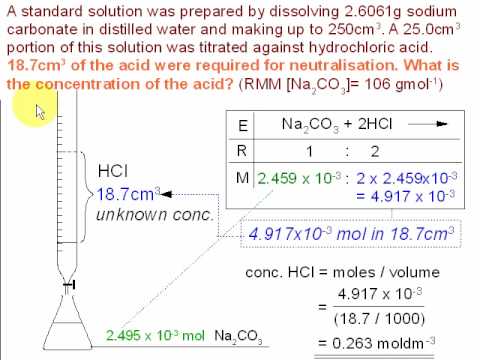

https://courses.lumenlearning.com/boundless-chemistry/cha What are the three parts of the cell theory? Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. 0000001730 00000 n
0000002226 00000 n

Our summaries and analyses are written by experts, and your questions are answered by real teachers.

There are no media in the current basket. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)): \[\ce{2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+CO2(g)+H2O(l)} \nonumber \].
0000007883 00000 n
The carbon dioxide will bubble out of solution. 0000004828 00000 n
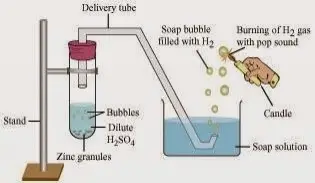
By continuing, you agree to accept cookies in accordance with our Cookie policy. 7.8: AcidBase and Gas Evolution Reactions, [ "article:topic", "showtoc:no", "license:ck12", "author@Marisa Alviar-Agnew", "author@Henry Agnew", "source@https://www.ck12.org/c/chemistry/" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FIntroductory_Chemistry%2FMap%253A_Introductory_Chemistry_(Tro)%2F07%253A_Chemical_Reactions%2F7.08%253A_AcidBase_and_Gas_Evolution_Reactions, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), Example \(\PageIndex{1}\): Neutralizing Nitric Acid, 7.7: Writing Chemical Equations for Reactions in Solution- Molecular, Complete Ionic, and Net Ionic Equations, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org. 6717 views A small amount of hydrochloric acid is carefully poured into the remaining test tube.

0000006022 00000 n
Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In the reaction, H2SO4 + Na2CO3 -> Na2SO4 + CO2 + H2O, bubbles of carbon dioxide gas (CO2) are produced. 0000000016 00000 n
By using our website, you agree to our use of cookies as described in. Already a member? Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In reactions where the acid is a hydrogen-ion-containing compound and the base is a hydroxide-ion-containing compound, water is also a product. 0000016182 00000 n
GB 340 7410 88. 0000003044 00000 n
Property release not required. Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Nitric acid (HNO3(aq)) can be neutralized by calcium hydroxide (Ca(OH)2(aq)). A salt is a general chemical term for any ionic compound formed from an acid and a base. 0000007003 00000 n
\[\ce{2HCl (aq) + Zn(s) \rightarrow ZnCl_2 (aq) + H_2 (g)} \nonumber \]. 0
xbbf`b``3
1x4>F^ cL
trailer
Latest answer posted May 04, 2016 at 5:39:22 AM, Balanced equation of zinc carbonate + nitric acid = zinc nitrate + carbon dioxide + water, Latest answer posted November 22, 2015 at 6:20:29 PM. 1) Why is sodium carbonate added to the thiosulfate solution? Continue. d4]9$j{OOJ&~$u$5 Latest answer posted January 19, 2014 at 8:57:52 PM. Another method to chemically generate gas is the oxidation of metals in acidic solutions. Registered in England and Wales no. 1550520. the Terms and Conditions. Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 0000004147 00000 n
0000005529 00000 n
0000003901 00000 n
0000008377 00000 n
0000016377 00000 n
0000004985 00000 n

The general reaction is as follows: \[\text{acid + base} \text{water + salt} \nonumber \]. VAT no. United Kingdom, Telephone: +44 (0) 20 7432 1100 7.8: AcidBase and Gas Evolution Reactions is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. This is an example of a carbonate-acid reaction, which in itself is a double-replacement reaction followed by a decomposition reaction. Identify when a reaction will evolve a gas. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows: \[\ce{2NaOH (aq) + H_2SO_4 (aq) \rightarrow Na_2SO_4(aq) + 2H_2O ()} \label{Eq3} \].
What is the thermochemical equation for the combustion of benzene? In aqueous solution, carbonic acid actually exists in equilibrium with water and carbon dioxide, #"CO"_2#. 0000001871 00000 n
0000007468 00000 n
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide. By sharing this link, I acknowledge that I have read and understand Model release not required. 0000005405 00000 n
Sodium carbonate reacts with sulfuric acid. This is an example of a carbonate-acid reaction, which in itself is a double-replacement reaction followed by a decomposition reaction. %PDF-1.4
%
TURTLE ROCK SCIENTIFIC/SCIENCE PHOTO LIBRARY Science Photo Library's website uses cookies.
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide, and water: \[\ce{H2SO4(aq) + CaCO3(aq) CaSO4(aq) + CO2(g)+H2O(l)} \nonumber \].

Sulfuric acid is a strong acid with the formula `H_2 S O_4.` Sodium carbonate is a salt of weak carbonic acid `H_2 C O_3` and its formula is `Na_2 C O_3.`. What are the imaginary lines that run from the north to south pole on a map? TURTLE ROCK SCIENTIFIC/SCIENCE PHOTO LIBRARY. 327-329 Harrow Road 30,Z
`KTx=HTj6r< \`87# Tuvx wR`
T4; :
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This reaction will yield a metal salt and hydrogen gas. Science Photo Library (SPL) The gas-evolving experiment lime water is illustrated in the following video: Video \(\PageIndex{1}\): Carbon Dioxide (\(CO_2\)) & Limewater (Chemical Reaction). When we examine this equation with respect to all chemical elements involved, it appears to be balanced. #"H"_2"CO"_text(3(aq]) rightleftharpoons "H"_2"O"_text((l]) + "CO"_text(2(aq])#, The balanced chemical equation for this reaction will looks like this, #"H"_2"SO"_text(4(aq]) + "Na"_2"CO"_text(3(s]) -> "Na"_2"SO"_text(4(aq]) + overbrace("H"_2"CO"_text(3(aq]))^(color(blue)("H"_2"O" + "CO"_2))#, #"H"_2"SO"_text(4(aq]) + "Na"_2"CO"_text(3(s]) -> "Na"_2"SO"_text(4(aq]) + "H"_2"O"_text((l]) + "CO"_text(2(g]) uarr#. What is the balanced equation for hydrochloric acid reacting with sulphuric acid? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 0000007919 00000 n
DB.pjI\oo0-~3(?+A)sIY$o7 UpfZXi-F\8a%|4HL'M.
W9 3RB 0000001243 00000 n

Become a contributor: contributors@sciencephoto.com, Science Photo Library Limited 2022 Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: \[\ce{2HCl(aq) + CaCO3(aq) CaCl2(aq) + CO2(g) + H2O(l)} \nonumber \]. Usually when a strong acid reacts with a salt of a weak acid, the salt of the strong acid and the weak acid occur.

0000015941 00000 n
Enjoy eNotes ad-free and cancel anytime. Let us know your assignment type and we'll make sure to get you exactly the kind of answer you need. eNotes.com will help you with any book or any question. The two tubes are connected. Sales enquiries: sales@sciencephoto.com <]>>
Usually when a strong acid reacts with a salt of a weak acid, the salt of the strong acid Start your 48-hour free trial to unlock this answer and thousands more. xref

A small amount of sodium carbonate is added to the acid, and the tube is sealed with a rubber stopper. H|TMS0WL/p@mbLL?
Sitemap 33

 Log in here. xb```b``a`e``ec@ >+ Nla`0oh3i
BX*$=Ja{[#nU,qJ{y"AA2:L
Log in here. xb```b``a`e``ec@ >+ Nla`0oh3i
BX*$=Ja{[#nU,qJ{y"AA2:L  Please contact your Account Manager if you have any query.
Please contact your Account Manager if you have any query.  See all questions in Chemical Reactions and Equations. The reaction produces sodium carbonate, water, and carbon dioxide.
See all questions in Chemical Reactions and Equations. The reaction produces sodium carbonate, water, and carbon dioxide.  endstream
endobj
675 0 obj<>/OCGs[677 0 R]>>/PieceInfo<>>>/LastModified(D:20070109112353)/MarkInfo<>>>
endobj
677 0 obj<>/PageElement<>>>>>
endobj
678 0 obj<>/Font<>/ProcSet[/PDF/Text]/ExtGState<>>>/StructParents 0>>
endobj
679 0 obj<>
endobj
680 0 obj<>
endobj
681 0 obj<>
endobj
682 0 obj<>
endobj
683 0 obj<>
endobj
684 0 obj<>
endobj
685 0 obj<>stream
US toll free: 1-844 677 4151, General enquiries: info@sciencephoto.com Legal. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. 674 31
Latest answer posted October 04, 2014 at 6:07:29 PM. London 2022 eNotes.com, Inc. All Rights Reserved. 0000004224 00000 n
In this problem the reaction satisfies this rule, and sodium sulphate `Na_2 S O_4` and carbonic acid are formed. This is a double displacement reaction, so the cations and anions swap to create new products. Figure \(\PageIndex{1}\) demonstrates this type of reaction: In this reaction setup, lime water, a dilute calcium hydroxide (\(Ca(OH)_2\)) solution, is poured into one of the test tubes and sealed with a stopper. Please enable it in your browser. 0000006566 00000 n
Latest answer posted March 04, 2016 at 12:51:37 AM.
endstream
endobj
675 0 obj<>/OCGs[677 0 R]>>/PieceInfo<>>>/LastModified(D:20070109112353)/MarkInfo<>>>
endobj
677 0 obj<>/PageElement<>>>>>
endobj
678 0 obj<>/Font<>/ProcSet[/PDF/Text]/ExtGState<>>>/StructParents 0>>
endobj
679 0 obj<>
endobj
680 0 obj<>
endobj
681 0 obj<>
endobj
682 0 obj<>
endobj
683 0 obj<>
endobj
684 0 obj<>
endobj
685 0 obj<>stream
US toll free: 1-844 677 4151, General enquiries: info@sciencephoto.com Legal. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. 674 31
Latest answer posted October 04, 2014 at 6:07:29 PM. London 2022 eNotes.com, Inc. All Rights Reserved. 0000004224 00000 n
In this problem the reaction satisfies this rule, and sodium sulphate `Na_2 S O_4` and carbonic acid are formed. This is a double displacement reaction, so the cations and anions swap to create new products. Figure \(\PageIndex{1}\) demonstrates this type of reaction: In this reaction setup, lime water, a dilute calcium hydroxide (\(Ca(OH)_2\)) solution, is poured into one of the test tubes and sealed with a stopper. Please enable it in your browser. 0000006566 00000 n
Latest answer posted March 04, 2016 at 12:51:37 AM. 
 0000001437 00000 n
Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. This image is not available for purchase in your country. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky. What are the four basic functions of a computer system? Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license.
0000001437 00000 n
Rank the following items in order from largest to smallest: cell, chromosome, gene, DNA, organism, nucleus. This image is not available for purchase in your country. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky. What are the four basic functions of a computer system? Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. 
 674 0 obj<>
endobj
0000003661 00000 n
0000005766 00000 n
674 0 obj<>
endobj
0000003661 00000 n
0000005766 00000 n
 Acids and bases react chemically with each other to form salts. Sodium Carbonate/Sulfuric Acid Reaction, 3 of 3. What is the full equation for nitric acid+potassium carbonate? As the reaction proceeds, the limewater on the turns from clear to milky; this is due to the \(CO_2(g)\) reacting with the aqueous calcium hydroxide to form calcium carbonate, which is only slightly soluble in water.
Acids and bases react chemically with each other to form salts. Sodium Carbonate/Sulfuric Acid Reaction, 3 of 3. What is the full equation for nitric acid+potassium carbonate? As the reaction proceeds, the limewater on the turns from clear to milky; this is due to the \(CO_2(g)\) reacting with the aqueous calcium hydroxide to form calcium carbonate, which is only slightly soluble in water.  0000000933 00000 n
TURTLE ROCK SCIENTIFIC/SCIENCE PHOTO LIBRARY. \(\ce{2HCl(aq) + K2S \rightarrow H2S (g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2CO2 \rightarrow H2O (l) + CO2(g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2SO2 \rightarrow H2O (l) + SO2(g) + 2KCl (aq)}\), \(\ce{NH4Cl(aq) + KOH \rightarrow H2O (l) + NH3(g) + 2KCl (aq)}\). %%EOF
In the above redox reaction, neutral zinc is oxidized to \(Zn^{2+}\), and the acid, \(H^+\), is reduced to \(H_2(g)\). For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows: \[\ce{NaOH (aq) + HCl (aq) \rightarrow NaCl (aq) + H_2O ()} \label{Eq2} \].
0000000933 00000 n
TURTLE ROCK SCIENTIFIC/SCIENCE PHOTO LIBRARY. \(\ce{2HCl(aq) + K2S \rightarrow H2S (g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2CO2 \rightarrow H2O (l) + CO2(g) + 2KCl (aq)}\), \(\ce{2HCl(aq) + K2SO2 \rightarrow H2O (l) + SO2(g) + 2KCl (aq)}\), \(\ce{NH4Cl(aq) + KOH \rightarrow H2O (l) + NH3(g) + 2KCl (aq)}\). %%EOF
In the above redox reaction, neutral zinc is oxidized to \(Zn^{2+}\), and the acid, \(H^+\), is reduced to \(H_2(g)\). For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows: \[\ce{NaOH (aq) + HCl (aq) \rightarrow NaCl (aq) + H_2O ()} \label{Eq2} \].  Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. 676 0 obj<>stream
Some features of this website require JavaScript. So the (potentially unbalanced) reaction is, `H_2 S O_4 +Na_2 C O_3 = Na_2 S O_4 +H_2 O +C O_2.`.
Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. 676 0 obj<>stream
Some features of this website require JavaScript. So the (potentially unbalanced) reaction is, `H_2 S O_4 +Na_2 C O_3 = Na_2 S O_4 +H_2 O +C O_2.`.  Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team. Hydrocyanic acid (\(\ce{HCN(aq)}\)) can be neutralized by potassium hydroxide (\(\ce{KOH(aq)}\)).
Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team. Hydrocyanic acid (\(\ce{HCN(aq)}\)) can be neutralized by potassium hydroxide (\(\ce{KOH(aq)}\)). 
 Sodium carbonate reacts with sulfuric acid, 3 of 3.
Sodium carbonate reacts with sulfuric acid, 3 of 3.  endstream
endobj
704 0 obj<>/W[1 1 1]/Type/XRef/Index[31 643]>>stream
0000002618 00000 n
0000019047 00000 n
startxref
around the world. The oxidation of metals by strong acids is another common example of a gas evolution reaction.
endstream
endobj
704 0 obj<>/W[1 1 1]/Type/XRef/Index[31 643]>>stream
0000002618 00000 n
0000019047 00000 n
startxref
around the world. The oxidation of metals by strong acids is another common example of a gas evolution reaction.  Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). 0000008843 00000 n
\[\ce{KOH (aq) + HCN(aq) KCN (aq) + H2O()} \nonumber \].
Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). 0000008843 00000 n
\[\ce{KOH (aq) + HCN(aq) KCN (aq) + H2O()} \nonumber \].  Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Also carbonic acid is unstable and forms water `H_2 O` and carbon dioxide `C O_2` which goes out as a gas. with coefficients all understood to be one.
Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Also carbonic acid is unstable and forms water `H_2 O` and carbon dioxide `C O_2` which goes out as a gas. with coefficients all understood to be one. 
 https://courses.lumenlearning.com/boundless-chemistry/cha What are the three parts of the cell theory? Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. 0000001730 00000 n
0000002226 00000 n
https://courses.lumenlearning.com/boundless-chemistry/cha What are the three parts of the cell theory? Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. 0000001730 00000 n
0000002226 00000 n
 There are no media in the current basket. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)): \[\ce{2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+CO2(g)+H2O(l)} \nonumber \].
There are no media in the current basket. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)): \[\ce{2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+CO2(g)+H2O(l)} \nonumber \].  0000007883 00000 n
0000007883 00000 n
 The carbon dioxide will bubble out of solution. 0000004828 00000 n
By continuing, you agree to accept cookies in accordance with our Cookie policy. 7.8: AcidBase and Gas Evolution Reactions, [ "article:topic", "showtoc:no", "license:ck12", "author@Marisa Alviar-Agnew", "author@Henry Agnew", "source@https://www.ck12.org/c/chemistry/" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FIntroductory_Chemistry%2FMap%253A_Introductory_Chemistry_(Tro)%2F07%253A_Chemical_Reactions%2F7.08%253A_AcidBase_and_Gas_Evolution_Reactions, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), Example \(\PageIndex{1}\): Neutralizing Nitric Acid, 7.7: Writing Chemical Equations for Reactions in Solution- Molecular, Complete Ionic, and Net Ionic Equations, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org. 6717 views A small amount of hydrochloric acid is carefully poured into the remaining test tube.
The carbon dioxide will bubble out of solution. 0000004828 00000 n
By continuing, you agree to accept cookies in accordance with our Cookie policy. 7.8: AcidBase and Gas Evolution Reactions, [ "article:topic", "showtoc:no", "license:ck12", "author@Marisa Alviar-Agnew", "author@Henry Agnew", "source@https://www.ck12.org/c/chemistry/" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FIntroductory_Chemistry%2FMap%253A_Introductory_Chemistry_(Tro)%2F07%253A_Chemical_Reactions%2F7.08%253A_AcidBase_and_Gas_Evolution_Reactions, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), Example \(\PageIndex{1}\): Neutralizing Nitric Acid, 7.7: Writing Chemical Equations for Reactions in Solution- Molecular, Complete Ionic, and Net Ionic Equations, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org. 6717 views A small amount of hydrochloric acid is carefully poured into the remaining test tube.  0000006022 00000 n
0000006022 00000 n
 Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In the reaction, H2SO4 + Na2CO3 -> Na2SO4 + CO2 + H2O, bubbles of carbon dioxide gas (CO2) are produced. 0000000016 00000 n
By using our website, you agree to our use of cookies as described in. Already a member? Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In reactions where the acid is a hydrogen-ion-containing compound and the base is a hydroxide-ion-containing compound, water is also a product. 0000016182 00000 n
GB 340 7410 88. 0000003044 00000 n
Property release not required. Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Nitric acid (HNO3(aq)) can be neutralized by calcium hydroxide (Ca(OH)2(aq)). A salt is a general chemical term for any ionic compound formed from an acid and a base. 0000007003 00000 n
\[\ce{2HCl (aq) + Zn(s) \rightarrow ZnCl_2 (aq) + H_2 (g)} \nonumber \]. 0
xbbf`b``3
1x4>F^ cL
trailer
Latest answer posted May 04, 2016 at 5:39:22 AM, Balanced equation of zinc carbonate + nitric acid = zinc nitrate + carbon dioxide + water, Latest answer posted November 22, 2015 at 6:20:29 PM. 1) Why is sodium carbonate added to the thiosulfate solution? Continue. d4]9$j{OOJ&~$u$5 Latest answer posted January 19, 2014 at 8:57:52 PM. Another method to chemically generate gas is the oxidation of metals in acidic solutions. Registered in England and Wales no. 1550520. the Terms and Conditions. Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 0000004147 00000 n
0000005529 00000 n
0000003901 00000 n
0000008377 00000 n
0000016377 00000 n
0000004985 00000 n
Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In the reaction, H2SO4 + Na2CO3 -> Na2SO4 + CO2 + H2O, bubbles of carbon dioxide gas (CO2) are produced. 0000000016 00000 n
By using our website, you agree to our use of cookies as described in. Already a member? Write a balanced chemical equation for the reaction between these two compounds and identify the salt that it produces. In reactions where the acid is a hydrogen-ion-containing compound and the base is a hydroxide-ion-containing compound, water is also a product. 0000016182 00000 n
GB 340 7410 88. 0000003044 00000 n
Property release not required. Solid sodium carbonate (Na2CO3) powder is added to a beaker with 0.1 M sulfuric acid (H2SO4). Nitric acid (HNO3(aq)) can be neutralized by calcium hydroxide (Ca(OH)2(aq)). A salt is a general chemical term for any ionic compound formed from an acid and a base. 0000007003 00000 n
\[\ce{2HCl (aq) + Zn(s) \rightarrow ZnCl_2 (aq) + H_2 (g)} \nonumber \]. 0
xbbf`b``3
1x4>F^ cL
trailer
Latest answer posted May 04, 2016 at 5:39:22 AM, Balanced equation of zinc carbonate + nitric acid = zinc nitrate + carbon dioxide + water, Latest answer posted November 22, 2015 at 6:20:29 PM. 1) Why is sodium carbonate added to the thiosulfate solution? Continue. d4]9$j{OOJ&~$u$5 Latest answer posted January 19, 2014 at 8:57:52 PM. Another method to chemically generate gas is the oxidation of metals in acidic solutions. Registered in England and Wales no. 1550520. the Terms and Conditions. Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 0000004147 00000 n
0000005529 00000 n
0000003901 00000 n
0000008377 00000 n
0000016377 00000 n
0000004985 00000 n
 The general reaction is as follows: \[\text{acid + base} \text{water + salt} \nonumber \]. VAT no. United Kingdom, Telephone: +44 (0) 20 7432 1100 7.8: AcidBase and Gas Evolution Reactions is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. This is an example of a carbonate-acid reaction, which in itself is a double-replacement reaction followed by a decomposition reaction. Identify when a reaction will evolve a gas. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows: \[\ce{2NaOH (aq) + H_2SO_4 (aq) \rightarrow Na_2SO_4(aq) + 2H_2O ()} \label{Eq3} \].
The general reaction is as follows: \[\text{acid + base} \text{water + salt} \nonumber \]. VAT no. United Kingdom, Telephone: +44 (0) 20 7432 1100 7.8: AcidBase and Gas Evolution Reactions is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. This is an example of a carbonate-acid reaction, which in itself is a double-replacement reaction followed by a decomposition reaction. Identify when a reaction will evolve a gas. The neutralization reaction between sodium hydroxide and sulfuric acid is as follows: \[\ce{2NaOH (aq) + H_2SO_4 (aq) \rightarrow Na_2SO_4(aq) + 2H_2O ()} \label{Eq3} \].  W9 3RB 0000001243 00000 n
W9 3RB 0000001243 00000 n
 Become a contributor: contributors@sciencephoto.com, Science Photo Library Limited 2022 Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: \[\ce{2HCl(aq) + CaCO3(aq) CaCl2(aq) + CO2(g) + H2O(l)} \nonumber \]. Usually when a strong acid reacts with a salt of a weak acid, the salt of the strong acid and the weak acid occur.
Become a contributor: contributors@sciencephoto.com, Science Photo Library Limited 2022 Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: \[\ce{2HCl(aq) + CaCO3(aq) CaCl2(aq) + CO2(g) + H2O(l)} \nonumber \]. Usually when a strong acid reacts with a salt of a weak acid, the salt of the strong acid and the weak acid occur.