This particular one is 1-ethoxypropane or ethyl propyl ether. Benzaldehyde on reaction with acetophenone in the presence of sodium sodium hydroxide sodium gives, Introduction to Three Dimensional Geometry. PageIndex: ["{1.1. This page describes the reaction between alcohols and metallic sodium,and introduces the properties of the alkoxide that is formed. The solution is strongly alkaline because ethoxide ions are Brnsted-Lowry bases and remove hydrogen ions from water molecules to produce hydroxide ions, which increase the pH. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org.


If the solution is evaporated carefully to dryness, then the sodium hydroxide (\(NaOH\)) is left behind as a white solid.

Legal. This reaction is known as the. a good method of synthesizing ethers in the lab. The reason that the ethoxide formula is written with the oxygen on the right unlike the hydroxide ion is simply a matter of clarity.

Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the reaction between sodium and water - something you have probably known about for years.


Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. MathJax.Hub.Config({
/*
If you add water to sodium ethoxide, it dissolves to give a colorless solution with a high pH. The solution is strongly alkaline because ethoxide ions are.

\[ CH_3CH_2CH_2OH + CH_3CH_2Br \rightarrow CH_3CH_2CH_2OCH_2CH_3 + Br^- \]. This reaction is known as the Williamson Ether Synthesis and is a good method of synthesizing ethers in the lab. If you spill some sodium on the bench or have a small amount left over from a reaction you cannot simply dispose of it in the sink.
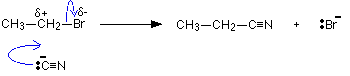
We will look at the reaction between sodium and ethanol as being typical, but you could substitute any other alcohol and the reaction would be the same. This page titled The Reaction Between Alcohols and Sodium is shared under a not declared license and was authored, remixed, and/or curated by Jim Clark. The only difference is that where there was a hydrogen atom at the right-hand end of the product molecule, an alkyl group is now present.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. equationNumbers: {
Get Answer to any question, just click a photo and upload the photo and get the answer completely free, in this question we need to discuss reaction of propanamide with atomic padam part of the improvement will give you what will be there when what is the solution is going to be American version to basically a mind when converted it known as declaration date sheet 2020 BA propanamide to you will be the carbon 13.288828 full form validation with a need to take it was as well and present is the mean agent is linked with which are performing from, 2 degree doubt will be separated on the following product which near me to buy it is important that we provide a 3rd year 2002 the major product which one degree amine what is going to be the Spanish we are going to get we are going to get form of the additional product generated which will be of any form and water molecule will there and anywhere collection will be there at of additional product which are not important over here we need to identify the maximum product that is being generated present to be our earth and our thank you, Click here to get PDF DOWNLOAD for all questions and answers of this chapter - NEET PREVIOUS YEAR ENGLISH Class 12 RE-NEET 2020.

If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. A nucleophile is a chemical species that carries a negative or partial negative charge that it uses to attack positive centers in other molecules or ions. Macros: {
Ethanol is, therefore, used to dissolve small quantities of waste sodium. The ethoxide ion behaves in exactly the same way. + n }

},
The anion component is an alkoxide. Propanamide on reaction with bromine in aqueous NaOH gives: Sodium ethoxide can be prepared by the reaction of ethanol with aqueous sodium hydroxide. formatNumber: function (n) { return 1.1 + '.' \[2H_2O_{(l)} + 2Na_{(s)} \rightarrow 2OH^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\]. Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.

Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the, We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. It tends to react explosively with the water - and comes flying back out at you again! autoNumber: "all",
This particular one is 1-ethoxypropane or ethyl propyl ether. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
Sodium hydroxide contains. The hydroxide ions replace the halogen atom. The anion component is an, If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. TeX: {
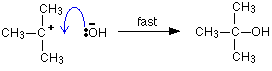
You will find the same thing happens when you write formulae for organic salts like sodium ethanoate, for example. }
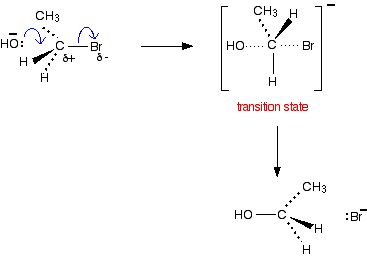
If you knew the mechanism for the hydroxide ion reaction, you could work out exactly what happens in the reaction between a halogenoalkane and ethoxide ion.
Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12.



The only difference is that where there was a hydrogen atom at the right-hand end of the product molecule, an alkyl group is now present. \[ CH_3CH_2CH_2Br + OH^- \rightarrow CH_3CH_2CH_2OH + Br^-\]. If you write it the other way around, it doesn't immediately look as if it comes from ethanol.
UndefinedNameError: reference to undefined name 'ContribClark', (Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Reactivity_of_Alcohols/The_Reaction_Between_Alcohols_and_Sodium), /content/body/div[4]/ul/li/span, line 1, column 1, Oxidation by PCC (pyridinium chlorochromate), The Reaction between Sodium Metal and Ethanol, status page at https://status.libretexts.org.

[CDATA[*/
We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. });
The ethoxide ion behaves in exactly the same way.

Sodium hydroxide contains \(OH^-\) ions; sodium ethoxide contains \(CH_3CH_2O^-\) ions.


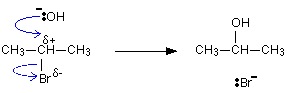
Hydroxide ions are good nucleophiles, and you may have come across the reaction between a halogenoalkane (also called a haloalkane or alkyl halide) and sodium hydroxide solution. There are two simple uses for this reaction: If you add water to sodium ethoxide, it dissolves to give a colorless solution with a high pH. \[2CH_3CH_2OH_{(l)} + 2Na_{(s)} \rightarrow 2CH_3CH_2O^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\].

\[ CH_3CH_2O^- + H_2O \rightarrow CH_3CH_2OH + OH^-\].
It reacts much more gently with ethanol. If you knew the mechanism for the hydroxide ion reaction, you could work out exactly what happens in the reaction between a halogenoalkane and ethoxide ion.
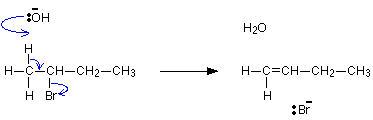
/*]]>*/. In this case, an alcohol is formed. Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. The solution formed can be washed away without problems (provided you remember that sodium ethoxide is strongly alkaline - see below).
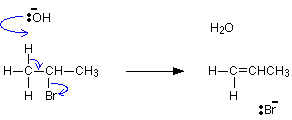
#1}",1]
[ "article:topic", "authorname:clarkj", "showtoc:no" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FOrganic_Chemistry%2FSupplemental_Modules_(Organic_Chemistry)%2FAlcohols%2FReactivity_of_Alcohols%2FThe_Reaction_Between_Alcohols_and_Sodium, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless solution of sodium ethoxide: \(CH_3CH_2ONa\).
Sitemap 15

 If the solution is evaporated carefully to dryness, then the sodium hydroxide (\(NaOH\)) is left behind as a white solid.
If the solution is evaporated carefully to dryness, then the sodium hydroxide (\(NaOH\)) is left behind as a white solid. 
 Legal. This reaction is known as the. a good method of synthesizing ethers in the lab. The reason that the ethoxide formula is written with the oxygen on the right unlike the hydroxide ion is simply a matter of clarity.
Legal. This reaction is known as the. a good method of synthesizing ethers in the lab. The reason that the ethoxide formula is written with the oxygen on the right unlike the hydroxide ion is simply a matter of clarity. 
 Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the reaction between sodium and water - something you have probably known about for years.
Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the reaction between sodium and water - something you have probably known about for years. 
 Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. MathJax.Hub.Config({
/*
Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. MathJax.Hub.Config({
/*  \[ CH_3CH_2CH_2OH + CH_3CH_2Br \rightarrow CH_3CH_2CH_2OCH_2CH_3 + Br^- \]. This reaction is known as the Williamson Ether Synthesis and is a good method of synthesizing ethers in the lab. If you spill some sodium on the bench or have a small amount left over from a reaction you cannot simply dispose of it in the sink.
\[ CH_3CH_2CH_2OH + CH_3CH_2Br \rightarrow CH_3CH_2CH_2OCH_2CH_3 + Br^- \]. This reaction is known as the Williamson Ether Synthesis and is a good method of synthesizing ethers in the lab. If you spill some sodium on the bench or have a small amount left over from a reaction you cannot simply dispose of it in the sink.  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. equationNumbers: {
Get Answer to any question, just click a photo and upload the photo and get the answer completely free, in this question we need to discuss reaction of propanamide with atomic padam part of the improvement will give you what will be there when what is the solution is going to be American version to basically a mind when converted it known as declaration date sheet 2020 BA propanamide to you will be the carbon 13.288828 full form validation with a need to take it was as well and present is the mean agent is linked with which are performing from, 2 degree doubt will be separated on the following product which near me to buy it is important that we provide a 3rd year 2002 the major product which one degree amine what is going to be the Spanish we are going to get we are going to get form of the additional product generated which will be of any form and water molecule will there and anywhere collection will be there at of additional product which are not important over here we need to identify the maximum product that is being generated present to be our earth and our thank you, Click here to get PDF DOWNLOAD for all questions and answers of this chapter - NEET PREVIOUS YEAR ENGLISH Class 12 RE-NEET 2020.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. equationNumbers: {
Get Answer to any question, just click a photo and upload the photo and get the answer completely free, in this question we need to discuss reaction of propanamide with atomic padam part of the improvement will give you what will be there when what is the solution is going to be American version to basically a mind when converted it known as declaration date sheet 2020 BA propanamide to you will be the carbon 13.288828 full form validation with a need to take it was as well and present is the mean agent is linked with which are performing from, 2 degree doubt will be separated on the following product which near me to buy it is important that we provide a 3rd year 2002 the major product which one degree amine what is going to be the Spanish we are going to get we are going to get form of the additional product generated which will be of any form and water molecule will there and anywhere collection will be there at of additional product which are not important over here we need to identify the maximum product that is being generated present to be our earth and our thank you, Click here to get PDF DOWNLOAD for all questions and answers of this chapter - NEET PREVIOUS YEAR ENGLISH Class 12 RE-NEET 2020. 
 If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. A nucleophile is a chemical species that carries a negative or partial negative charge that it uses to attack positive centers in other molecules or ions. Macros: {
Ethanol is, therefore, used to dissolve small quantities of waste sodium. The ethoxide ion behaves in exactly the same way. + n }
If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. A nucleophile is a chemical species that carries a negative or partial negative charge that it uses to attack positive centers in other molecules or ions. Macros: {
Ethanol is, therefore, used to dissolve small quantities of waste sodium. The ethoxide ion behaves in exactly the same way. + n }
 },
The anion component is an alkoxide. Propanamide on reaction with bromine in aqueous NaOH gives: Sodium ethoxide can be prepared by the reaction of ethanol with aqueous sodium hydroxide. formatNumber: function (n) { return 1.1 + '.' \[2H_2O_{(l)} + 2Na_{(s)} \rightarrow 2OH^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\]. Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
},
The anion component is an alkoxide. Propanamide on reaction with bromine in aqueous NaOH gives: Sodium ethoxide can be prepared by the reaction of ethanol with aqueous sodium hydroxide. formatNumber: function (n) { return 1.1 + '.' \[2H_2O_{(l)} + 2Na_{(s)} \rightarrow 2OH^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\]. Sodium ethoxide is just like sodium hydroxide, except that the hydrogen has been replaced by an ethyl group. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.  Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the, We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. It tends to react explosively with the water - and comes flying back out at you again! autoNumber: "all",
This particular one is 1-ethoxypropane or ethyl propyl ether. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
Although initially this appears as something new and complicated, in fact, it is exactly the same (apart from being a more gentle reaction) as the, We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. It tends to react explosively with the water - and comes flying back out at you again! autoNumber: "all",
This particular one is 1-ethoxypropane or ethyl propyl ether. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.  Sodium hydroxide contains. The hydroxide ions replace the halogen atom. The anion component is an, If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. TeX: {
Sodium hydroxide contains. The hydroxide ions replace the halogen atom. The anion component is an, If the solution is evaporated carefully to dryness, then sodium ethoxide (\(CH_3CH_2ONa\)) is left behind as a white solid. TeX: {
 Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12.
Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. 

 The only difference is that where there was a hydrogen atom at the right-hand end of the product molecule, an alkyl group is now present. \[ CH_3CH_2CH_2Br + OH^- \rightarrow CH_3CH_2CH_2OH + Br^-\]. If you write it the other way around, it doesn't immediately look as if it comes from ethanol.
The only difference is that where there was a hydrogen atom at the right-hand end of the product molecule, an alkyl group is now present. \[ CH_3CH_2CH_2Br + OH^- \rightarrow CH_3CH_2CH_2OH + Br^-\]. If you write it the other way around, it doesn't immediately look as if it comes from ethanol.  UndefinedNameError: reference to undefined name 'ContribClark', (Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Reactivity_of_Alcohols/The_Reaction_Between_Alcohols_and_Sodium), /content/body/div[4]/ul/li/span, line 1, column 1, Oxidation by PCC (pyridinium chlorochromate), The Reaction between Sodium Metal and Ethanol, status page at https://status.libretexts.org.
UndefinedNameError: reference to undefined name 'ContribClark', (Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Reactivity_of_Alcohols/The_Reaction_Between_Alcohols_and_Sodium), /content/body/div[4]/ul/li/span, line 1, column 1, Oxidation by PCC (pyridinium chlorochromate), The Reaction between Sodium Metal and Ethanol, status page at https://status.libretexts.org.  [CDATA[*/
We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. });
The ethoxide ion behaves in exactly the same way.
[CDATA[*/
We normally, of course, write the sodium hydroxide formed as \(NaOH\) rather than \(HONa\) - but that's the only difference. });
The ethoxide ion behaves in exactly the same way.  Sodium hydroxide contains \(OH^-\) ions; sodium ethoxide contains \(CH_3CH_2O^-\) ions.
Sodium hydroxide contains \(OH^-\) ions; sodium ethoxide contains \(CH_3CH_2O^-\) ions. 

 Hydroxide ions are good nucleophiles, and you may have come across the reaction between a halogenoalkane (also called a haloalkane or alkyl halide) and sodium hydroxide solution. There are two simple uses for this reaction: If you add water to sodium ethoxide, it dissolves to give a colorless solution with a high pH. \[2CH_3CH_2OH_{(l)} + 2Na_{(s)} \rightarrow 2CH_3CH_2O^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\].
Hydroxide ions are good nucleophiles, and you may have come across the reaction between a halogenoalkane (also called a haloalkane or alkyl halide) and sodium hydroxide solution. There are two simple uses for this reaction: If you add water to sodium ethoxide, it dissolves to give a colorless solution with a high pH. \[2CH_3CH_2OH_{(l)} + 2Na_{(s)} \rightarrow 2CH_3CH_2O^-_{(aq)} + 2Na^+_{(aq)} + H_{2(g)}\].  \[ CH_3CH_2O^- + H_2O \rightarrow CH_3CH_2OH + OH^-\].
\[ CH_3CH_2O^- + H_2O \rightarrow CH_3CH_2OH + OH^-\].  It reacts much more gently with ethanol. If you knew the mechanism for the hydroxide ion reaction, you could work out exactly what happens in the reaction between a halogenoalkane and ethoxide ion.
It reacts much more gently with ethanol. If you knew the mechanism for the hydroxide ion reaction, you could work out exactly what happens in the reaction between a halogenoalkane and ethoxide ion.  /*]]>*/. In this case, an alcohol is formed. Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. The solution formed can be washed away without problems (provided you remember that sodium ethoxide is strongly alkaline - see below).
/*]]>*/. In this case, an alcohol is formed. Two alkyl (or other hydrocarbon) groups bridged by an oxygen atom is called an ether. The solution formed can be washed away without problems (provided you remember that sodium ethoxide is strongly alkaline - see below).