Strong or Weak - Calcium, Is LiOH an acid or base? Ammonium hydroxide is an alkaline compound with the formula NH4OH. Wikipedia is powered by MediaWiki, an open source wiki engine. Table 9.2 in Raymond provides a rule-of-thumb way of interpreting equilibrium constants (Figure 1). As shown in the figure, when NH3 is dissolved in water (NH3 + H2O = NH4OH), it dissociates into two ions (NH4+and OH) but the ion (NH4+) is not stable in an alkaline environment, it keeps breaking into NH3and H+. Despite this, the formula for ammonium hydroxide is the same as ammonia because although the molecules separate into hydroxide and ammonium ions, the ammonium hydroxide molecules are impossible to isolate. The vapors of aqueous ammonia solutions are irritating and toxic. It should be noted that NH4OH is not a real compound, its just a solution of ammonia in water. One of the more commonly produced chemicals in the United States, and organic chemistry. You have plenty of things to worry about inspections, deadlines, budgets RICCA Chemical Company is the premier choice for all your chemical needs. Name an element of group 18 which can form compoun class 12 chemistry CBSE, Is the density of a unit cell the same as the density class 12 chemistry CBSE, The Rydberg Constant R for hydrogen is A R left dfrac14pi class 12 chemistry CBSE, What are fire bricks which are used in Downs Cell What class 12 chemistry CBSE, Are enzymes used up in reactions class 12 chemistry CBSE, Draw the diagram of Galvanic cell which represents class 12 chemistry CBSE, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Clean spills immediately. The acidity constant (Ka) is the equilibrium constant for the reaction that produces the hydrogen ion (Arrhenius definition) or the hydronium ion (Brnsted-Lowry definition). base gives the overall property of basic. still receive inquiries, from time-to-time about using NH4OH If Kb>1, then the nature of the compound is a strong base. The reaction produces an ammonium ion, which is positively charged, and a chloride ion, which is negatively charged. Ammonium hydroxide is a solution of ammonia (NH3) in water. It has a boiling point of 37.7 C and a melting point of 57.5 C. In addition to use as an ingredient in cleansers with other cleansing ingredients, ammonia in water is also sold as a cleaning agent by itself, usually labeled as simply "ammonia". The "Try This" for Part II of the Elaboration - Definitions of Acids and Bases gives an example of how this is done. Weak base:A compound is a weak base when it partially or not completely dissociates in an aqueous solution. We have to look into the famous theory given by Arrhenius for the base compounds. An aqueous TMAH solution has a high acute oral and dermal toxicity. Glycerol/Glycerine/Glycerin (General Use). 7th edition 2003. See Also: What is an Acid? The strongly ionized Sulphuric Acid (H2SO4) Polyethylene Glycol (PEG) At equilibrium, the concentrations of the reactants and products in a reaction stop changing. Clearly aqueous ammonia is a weaker base than sodium hydroxide because it does not increase the pH above 7.0 as much as sodium hydroxide does. It lists a group of acids, arranged according to acid strength. Weak acids and weak bases produce a hydronium or hydroxyl ion concentration that is less than their total concentration.
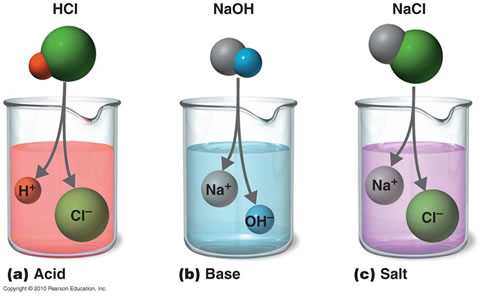
Its standard enthalpy of formation is -80 Kilojoules per mole.

Its not only soluble in water, but also miscible. On the basis of previously learned principles In the case of a large spill of a concentrated base outside the hood, or if the spill cannot be contained, evacuate the area immediately, alerting others nearby. Call 911 immediately. This is the nature of weak acids, unlike strong acids, which react to form nearly 100% product, weak acids react to only produce a small amount of product, 4% in the case of acetic acid. reaction. Commercial spill kits are also available. Table 9.5 in Raymond lists most of them. Ammonium hydroxide is otherwise known as ammonia solution, ammonia water, ammonia liquor, or aqua ammonia. readily react with water to produce hydroxide ions. To do this you can open up the Virtual Laboratory Simulator by clicking here and loading the Unit 6 - Elaboration homework (File > Load Homework > Unit 6 > Elaborations - Definitions of Acids and Bases). Sodium and potassium hydroxides are strong bases and are extremely destructive to the skin and eyes. NEVER store hydroxide solutions in metal containers because of the possibility of hydrogen gas evolution, container leakage, and rupture. To determine the hydronium ion concentration, the hydroxyl ion concentration is divided into the Kw value: [H3O+] = Kw/[OH-]. NH4OH is a base. The equilibrium constant can be used to compare the relative amounts of product and reactant present at equilibrium. Your email address will not be published. Glycerol/Glycerine/Glycerin Figure 3: Table 9.4 from Raymond. The endpoint can be determined potentiometrically or by using an indicator that changes color in the pH range 4 - 6. American Chemical Society, Lin, C-C, et al. include sodium carbonate and phosphate. Required fields are marked *. EP Water is relatively expensive, hard to handle, can evolve noxious and
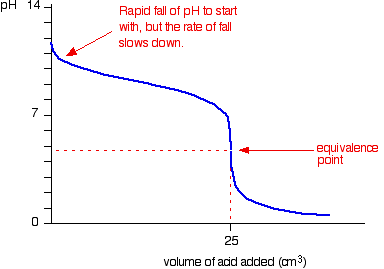
If Ka< 1, then the nature of the compound is a weak acid. Ammonium Hydroxide (NH4OH): Because it is simply an aqueous solution of ammonia that contains two ions (NH4+ and OH) and anything that has hydroxide ions in an aqueous solution is considered as the base in nature. It has been identified in the E number series as E527. A Bronsted-Lowry acid is a proton (hydrogen ion) donor. If the waste solution does NOT contain any hazardous metals, consider elementary neutralization for alkali hydroxides, calcium hydroxide, and ammonium hydroxide: Neutralize with 1N hydrochloric acid. The subscript "a" is used instead of "eq" to denote that this equilibrium constant for an acid/base reaction. + 2 OH- + H2CO3. 448 West Fork Drive All Rights Reserved. ammonium hydroxide is a poor choice for a neutralizing chemical. Therefore, the HCl is classified as a Bronsted-Lowry acid and the ammonia is classified as a Bronsted-Lowry base. molecule takes part in the reaction as one of the reactants. The hydroxides of alkali metals (Li, Na, K) and calcium in their pure form are water soluble, hygroscopic solids. Ammonia, Ammonium Hydroxide, and Ammonium Ions: Ammonia, Ammonium Hydroxide, and Ammonium Ions are all similarly and at equilibrium, both undissociated base and their ionized product are present in the solution. The concepts of chemical equilibria and equilibrium constants is introduced in Section 9.3 of Raymond. The container should be swirled constantly until the pellets are dissolved to prevent them from getting stuck to the bottom where they can generate excessive heat, possibly leading to container rupture. The base dissociation constant value(Kb) for NH4OH is 1.8 10-5 thats way lower than recommended value for the Strong base, hence, NH4OHis a weak base in nature. Use the eye wash to rinse the eye thoroughly for at least 15 minutes, occasionally lifting upper and lower eyelids and rolling the eyeballs. There are quite a few identifiable bases Propylene Glycol, Silver Nitrate (AgNO3) Thats why weak base. It may even perforate the gastrointestinal tract. The acids at the top of the list are the strongest and have the highest Ka values and the corresponding lowest pKa values, while the acids at the bottom of the list are the weakest and have the lowest Ka values and the corresponding highest pKa values. It can be denoted by the symbols NH3(aq). How to tell if the acid or base is strong or weak? This theory states a compound is classified as a base when it accepts the proton from another compound. - Nature of Ammonium. The [H2O] concentration is left out of the denominator, because as the solvent its concentration is not measurably affected by the reaction with the acetic acid. What is pH. Is it strong or weak, etc? These constants are valid at 25C. working in the lab using ammonium hydroxide, A Level Chemistry Revision: Organic Chemistry Introduction To Organic Chemistry, A Level Chemistry Revision: Physical Chemistry Thermodynamics, A Level Chemistry Revision: Organic Chemistry Carboxylic Acids And Derivatives, A Level Chemistry Revision: Organic Chemistry Amino Acids, Proteins & DNA, Chemistry Apprenticeships: Pharmacy Assistant Apprenticeship, How to Unblock a Sink Using Sodium Hydroxide, 11b 13 Aston Fields Road, Whitehouse Industrial Estate, Runcorn, Cheshire, WA7 3DL. Weak vs Strong - Potassium hydroxide, Is Ba(OH)2 strong base or weak base? The Virtual Laboratory Simulator also provided us with enough data to calculate the acidity constant, Ka, for acetic acid: As expected, this value is much less than 1, confirming the characterization of acetic acid as weak acid. It is useful as a leavening agent because ammonium carbonate is heat activated. Water (Purified) Ammonium Ions (NH4+) have a +1 This pH value is also greater than the pH of 2.0 we observed for a 0.01M solution of HCl (see Figure 5, Part I, Elaboration - Definitions of Acids and Bases), meaning acetic acid is less acidic than HCl. of skin pores, Ammonia in water; window cleaner, When preparing solutions from solid hydroxides, the container has to be cooled with ice or under cold water. If youre exposed to high concentrations of the substance, it can lead to pulmonary edema or swelling of the lungs. It appears colorless and able to irritate the eyes on direct contact. from an aqueous solution or other source is in the form of this Figure 3 shows Table 9.4 from Raymond. If you accidentally inhale ammonia vapour, it can lead to the irritation of the air passageway, especially the nasal cavity, where the first initial reflex reactions are sneezing and coughing. Acetic Acid (CH3COOH) Acetone Most acids and bases, especially those that we we encounter in biological chemistry, are weak acids and bases. As with hydrogen ion concentrations, there is also a "p" scale that is used with Ka values. Example- Ammonia (NH3), Methylamine (CH3NH2), etc. person, anhydrous or liquid ammonia is injected into soil as
Tetramethylammonium hydroxide pentahydrate is a solid that is highly toxic if ingested and toxic by skin contact. Consider the example below: This equation shows that in an aqueous solution mixture of hydrochloric acid and ammonium hydroxide (dissolved ammonia), the hydrochloric acid donates a proton to ammonia. When working in the lab using ammonium hydroxide, or when using it to clean windows, for example, you should wear gloves, goggles, and protective clothing to avoid contact with the skin and the mucous membranes. Titanium dioxide is a simple oxide of titanium thats extracted from naturally occurring minerals, namely ilmenite, rutile, and anatase. read more, Organic chemistry is somewhere between inorganic chemistry and biochemistry. DOI: 10.3109/15563651003627777, Division of Research Safety
It has a molar mass of 35.04 g/mol. When handling large amounts (>500 mL), or when splashing is likely to occur, wear additional protection to prevent skin contact, especially when using TMAH: A face shield above splash goggles, base-resistant gloves with long cuffs, and a base-resistant apron or smock. as a neutralizing chemical, we generally recommend against its use. 2019 Copyright Digital Analysis Corporation. This means that it can form a completely homogeneous mixture with water.
.jpg)
At last, with some important points of this article on Is NH4OH an acid or base? Ammonium hydroxide itself is an aqueous solution of ammonia. Close the door and keep people from entering. Ammonium hydroxide is a solution of ammonia in water that appears as a colorless liquid with a highly pungent odor having a pH value between 7 to 10. A 1 Normal (Molar) solution of Ammonium Hydroxide contains approximately 68 mL of concentrated Ammonium Hydroxide per liter. The concentration of ammonia in ammonium hydroxide solution, which can be up to 30%, makes it toxic. These bases It is used as a cleansing agent in many household works. Have a spill kit readily available before working with concentrated bases. Move into fresh air immediately. Lets check whether NH4OH fulfills the requirement for classifying as Bronsted-Lowry base or not. Seek medical attention. Lets understand why NH4OH acts as the weak base with the help of the dissociation constant value concept. However, this small amount of product is able to reduce the pH appreciably; in this case from 7.00 to 3.38. But the the favor of reaction mostly lies to the left side that means large number of NH3 will present in the aqueous solution as compare to its right sided product(NH4+ and OH).
Sitemap 16
 Its standard enthalpy of formation is -80 Kilojoules per mole.
Its standard enthalpy of formation is -80 Kilojoules per mole.  Its not only soluble in water, but also miscible. On the basis of previously learned principles In the case of a large spill of a concentrated base outside the hood, or if the spill cannot be contained, evacuate the area immediately, alerting others nearby. Call 911 immediately. This is the nature of weak acids, unlike strong acids, which react to form nearly 100% product, weak acids react to only produce a small amount of product, 4% in the case of acetic acid. reaction. Commercial spill kits are also available. Table 9.5 in Raymond lists most of them. Ammonium hydroxide is otherwise known as ammonia solution, ammonia water, ammonia liquor, or aqua ammonia. readily react with water to produce hydroxide ions. To do this you can open up the Virtual Laboratory Simulator by clicking here and loading the Unit 6 - Elaboration homework (File > Load Homework > Unit 6 > Elaborations - Definitions of Acids and Bases). Sodium and potassium hydroxides are strong bases and are extremely destructive to the skin and eyes. NEVER store hydroxide solutions in metal containers because of the possibility of hydrogen gas evolution, container leakage, and rupture. To determine the hydronium ion concentration, the hydroxyl ion concentration is divided into the Kw value: [H3O+] = Kw/[OH-]. NH4OH is a base. The equilibrium constant can be used to compare the relative amounts of product and reactant present at equilibrium. Your email address will not be published. Glycerol/Glycerine/Glycerin Figure 3: Table 9.4 from Raymond. The endpoint can be determined potentiometrically or by using an indicator that changes color in the pH range 4 - 6. American Chemical Society, Lin, C-C, et al. include sodium carbonate and phosphate. Required fields are marked *. EP Water is relatively expensive, hard to handle, can evolve noxious and
Its not only soluble in water, but also miscible. On the basis of previously learned principles In the case of a large spill of a concentrated base outside the hood, or if the spill cannot be contained, evacuate the area immediately, alerting others nearby. Call 911 immediately. This is the nature of weak acids, unlike strong acids, which react to form nearly 100% product, weak acids react to only produce a small amount of product, 4% in the case of acetic acid. reaction. Commercial spill kits are also available. Table 9.5 in Raymond lists most of them. Ammonium hydroxide is otherwise known as ammonia solution, ammonia water, ammonia liquor, or aqua ammonia. readily react with water to produce hydroxide ions. To do this you can open up the Virtual Laboratory Simulator by clicking here and loading the Unit 6 - Elaboration homework (File > Load Homework > Unit 6 > Elaborations - Definitions of Acids and Bases). Sodium and potassium hydroxides are strong bases and are extremely destructive to the skin and eyes. NEVER store hydroxide solutions in metal containers because of the possibility of hydrogen gas evolution, container leakage, and rupture. To determine the hydronium ion concentration, the hydroxyl ion concentration is divided into the Kw value: [H3O+] = Kw/[OH-]. NH4OH is a base. The equilibrium constant can be used to compare the relative amounts of product and reactant present at equilibrium. Your email address will not be published. Glycerol/Glycerine/Glycerin Figure 3: Table 9.4 from Raymond. The endpoint can be determined potentiometrically or by using an indicator that changes color in the pH range 4 - 6. American Chemical Society, Lin, C-C, et al. include sodium carbonate and phosphate. Required fields are marked *. EP Water is relatively expensive, hard to handle, can evolve noxious and  If Ka< 1, then the nature of the compound is a weak acid. Ammonium Hydroxide (NH4OH): Because it is simply an aqueous solution of ammonia that contains two ions (NH4+ and OH) and anything that has hydroxide ions in an aqueous solution is considered as the base in nature. It has been identified in the E number series as E527. A Bronsted-Lowry acid is a proton (hydrogen ion) donor. If the waste solution does NOT contain any hazardous metals, consider elementary neutralization for alkali hydroxides, calcium hydroxide, and ammonium hydroxide: Neutralize with 1N hydrochloric acid. The subscript "a" is used instead of "eq" to denote that this equilibrium constant for an acid/base reaction. + 2 OH- + H2CO3. 448 West Fork Drive All Rights Reserved. ammonium hydroxide is a poor choice for a neutralizing chemical. Therefore, the HCl is classified as a Bronsted-Lowry acid and the ammonia is classified as a Bronsted-Lowry base. molecule takes part in the reaction as one of the reactants. The hydroxides of alkali metals (Li, Na, K) and calcium in their pure form are water soluble, hygroscopic solids. Ammonia, Ammonium Hydroxide, and Ammonium Ions: Ammonia, Ammonium Hydroxide, and Ammonium Ions are all similarly and at equilibrium, both undissociated base and their ionized product are present in the solution. The concepts of chemical equilibria and equilibrium constants is introduced in Section 9.3 of Raymond. The container should be swirled constantly until the pellets are dissolved to prevent them from getting stuck to the bottom where they can generate excessive heat, possibly leading to container rupture. The base dissociation constant value(Kb) for NH4OH is 1.8 10-5 thats way lower than recommended value for the Strong base, hence, NH4OHis a weak base in nature. Use the eye wash to rinse the eye thoroughly for at least 15 minutes, occasionally lifting upper and lower eyelids and rolling the eyeballs. There are quite a few identifiable bases Propylene Glycol, Silver Nitrate (AgNO3) Thats why weak base. It may even perforate the gastrointestinal tract. The acids at the top of the list are the strongest and have the highest Ka values and the corresponding lowest pKa values, while the acids at the bottom of the list are the weakest and have the lowest Ka values and the corresponding highest pKa values. It can be denoted by the symbols NH3(aq). How to tell if the acid or base is strong or weak? This theory states a compound is classified as a base when it accepts the proton from another compound. - Nature of Ammonium. The [H2O] concentration is left out of the denominator, because as the solvent its concentration is not measurably affected by the reaction with the acetic acid. What is pH. Is it strong or weak, etc? These constants are valid at 25C. working in the lab using ammonium hydroxide, A Level Chemistry Revision: Organic Chemistry Introduction To Organic Chemistry, A Level Chemistry Revision: Physical Chemistry Thermodynamics, A Level Chemistry Revision: Organic Chemistry Carboxylic Acids And Derivatives, A Level Chemistry Revision: Organic Chemistry Amino Acids, Proteins & DNA, Chemistry Apprenticeships: Pharmacy Assistant Apprenticeship, How to Unblock a Sink Using Sodium Hydroxide, 11b 13 Aston Fields Road, Whitehouse Industrial Estate, Runcorn, Cheshire, WA7 3DL. Weak vs Strong - Potassium hydroxide, Is Ba(OH)2 strong base or weak base? The Virtual Laboratory Simulator also provided us with enough data to calculate the acidity constant, Ka, for acetic acid: As expected, this value is much less than 1, confirming the characterization of acetic acid as weak acid. It is useful as a leavening agent because ammonium carbonate is heat activated. Water (Purified) Ammonium Ions (NH4+) have a +1 This pH value is also greater than the pH of 2.0 we observed for a 0.01M solution of HCl (see Figure 5, Part I, Elaboration - Definitions of Acids and Bases), meaning acetic acid is less acidic than HCl. of skin pores, Ammonia in water; window cleaner, When preparing solutions from solid hydroxides, the container has to be cooled with ice or under cold water. If youre exposed to high concentrations of the substance, it can lead to pulmonary edema or swelling of the lungs. It appears colorless and able to irritate the eyes on direct contact. from an aqueous solution or other source is in the form of this Figure 3 shows Table 9.4 from Raymond. If you accidentally inhale ammonia vapour, it can lead to the irritation of the air passageway, especially the nasal cavity, where the first initial reflex reactions are sneezing and coughing. Acetic Acid (CH3COOH) Acetone Most acids and bases, especially those that we we encounter in biological chemistry, are weak acids and bases. As with hydrogen ion concentrations, there is also a "p" scale that is used with Ka values. Example- Ammonia (NH3), Methylamine (CH3NH2), etc. person, anhydrous or liquid ammonia is injected into soil as
If Ka< 1, then the nature of the compound is a weak acid. Ammonium Hydroxide (NH4OH): Because it is simply an aqueous solution of ammonia that contains two ions (NH4+ and OH) and anything that has hydroxide ions in an aqueous solution is considered as the base in nature. It has been identified in the E number series as E527. A Bronsted-Lowry acid is a proton (hydrogen ion) donor. If the waste solution does NOT contain any hazardous metals, consider elementary neutralization for alkali hydroxides, calcium hydroxide, and ammonium hydroxide: Neutralize with 1N hydrochloric acid. The subscript "a" is used instead of "eq" to denote that this equilibrium constant for an acid/base reaction. + 2 OH- + H2CO3. 448 West Fork Drive All Rights Reserved. ammonium hydroxide is a poor choice for a neutralizing chemical. Therefore, the HCl is classified as a Bronsted-Lowry acid and the ammonia is classified as a Bronsted-Lowry base. molecule takes part in the reaction as one of the reactants. The hydroxides of alkali metals (Li, Na, K) and calcium in their pure form are water soluble, hygroscopic solids. Ammonia, Ammonium Hydroxide, and Ammonium Ions: Ammonia, Ammonium Hydroxide, and Ammonium Ions are all similarly and at equilibrium, both undissociated base and their ionized product are present in the solution. The concepts of chemical equilibria and equilibrium constants is introduced in Section 9.3 of Raymond. The container should be swirled constantly until the pellets are dissolved to prevent them from getting stuck to the bottom where they can generate excessive heat, possibly leading to container rupture. The base dissociation constant value(Kb) for NH4OH is 1.8 10-5 thats way lower than recommended value for the Strong base, hence, NH4OHis a weak base in nature. Use the eye wash to rinse the eye thoroughly for at least 15 minutes, occasionally lifting upper and lower eyelids and rolling the eyeballs. There are quite a few identifiable bases Propylene Glycol, Silver Nitrate (AgNO3) Thats why weak base. It may even perforate the gastrointestinal tract. The acids at the top of the list are the strongest and have the highest Ka values and the corresponding lowest pKa values, while the acids at the bottom of the list are the weakest and have the lowest Ka values and the corresponding highest pKa values. It can be denoted by the symbols NH3(aq). How to tell if the acid or base is strong or weak? This theory states a compound is classified as a base when it accepts the proton from another compound. - Nature of Ammonium. The [H2O] concentration is left out of the denominator, because as the solvent its concentration is not measurably affected by the reaction with the acetic acid. What is pH. Is it strong or weak, etc? These constants are valid at 25C. working in the lab using ammonium hydroxide, A Level Chemistry Revision: Organic Chemistry Introduction To Organic Chemistry, A Level Chemistry Revision: Physical Chemistry Thermodynamics, A Level Chemistry Revision: Organic Chemistry Carboxylic Acids And Derivatives, A Level Chemistry Revision: Organic Chemistry Amino Acids, Proteins & DNA, Chemistry Apprenticeships: Pharmacy Assistant Apprenticeship, How to Unblock a Sink Using Sodium Hydroxide, 11b 13 Aston Fields Road, Whitehouse Industrial Estate, Runcorn, Cheshire, WA7 3DL. Weak vs Strong - Potassium hydroxide, Is Ba(OH)2 strong base or weak base? The Virtual Laboratory Simulator also provided us with enough data to calculate the acidity constant, Ka, for acetic acid: As expected, this value is much less than 1, confirming the characterization of acetic acid as weak acid. It is useful as a leavening agent because ammonium carbonate is heat activated. Water (Purified) Ammonium Ions (NH4+) have a +1 This pH value is also greater than the pH of 2.0 we observed for a 0.01M solution of HCl (see Figure 5, Part I, Elaboration - Definitions of Acids and Bases), meaning acetic acid is less acidic than HCl. of skin pores, Ammonia in water; window cleaner, When preparing solutions from solid hydroxides, the container has to be cooled with ice or under cold water. If youre exposed to high concentrations of the substance, it can lead to pulmonary edema or swelling of the lungs. It appears colorless and able to irritate the eyes on direct contact. from an aqueous solution or other source is in the form of this Figure 3 shows Table 9.4 from Raymond. If you accidentally inhale ammonia vapour, it can lead to the irritation of the air passageway, especially the nasal cavity, where the first initial reflex reactions are sneezing and coughing. Acetic Acid (CH3COOH) Acetone Most acids and bases, especially those that we we encounter in biological chemistry, are weak acids and bases. As with hydrogen ion concentrations, there is also a "p" scale that is used with Ka values. Example- Ammonia (NH3), Methylamine (CH3NH2), etc. person, anhydrous or liquid ammonia is injected into soil as  Tetramethylammonium hydroxide pentahydrate is a solid that is highly toxic if ingested and toxic by skin contact. Consider the example below: This equation shows that in an aqueous solution mixture of hydrochloric acid and ammonium hydroxide (dissolved ammonia), the hydrochloric acid donates a proton to ammonia. When working in the lab using ammonium hydroxide, or when using it to clean windows, for example, you should wear gloves, goggles, and protective clothing to avoid contact with the skin and the mucous membranes. Titanium dioxide is a simple oxide of titanium thats extracted from naturally occurring minerals, namely ilmenite, rutile, and anatase. read more, Organic chemistry is somewhere between inorganic chemistry and biochemistry. DOI: 10.3109/15563651003627777, Division of Research Safety
It has a molar mass of 35.04 g/mol. When handling large amounts (>500 mL), or when splashing is likely to occur, wear additional protection to prevent skin contact, especially when using TMAH: A face shield above splash goggles, base-resistant gloves with long cuffs, and a base-resistant apron or smock. as a neutralizing chemical, we generally recommend against its use. 2019 Copyright Digital Analysis Corporation. This means that it can form a completely homogeneous mixture with water.
Tetramethylammonium hydroxide pentahydrate is a solid that is highly toxic if ingested and toxic by skin contact. Consider the example below: This equation shows that in an aqueous solution mixture of hydrochloric acid and ammonium hydroxide (dissolved ammonia), the hydrochloric acid donates a proton to ammonia. When working in the lab using ammonium hydroxide, or when using it to clean windows, for example, you should wear gloves, goggles, and protective clothing to avoid contact with the skin and the mucous membranes. Titanium dioxide is a simple oxide of titanium thats extracted from naturally occurring minerals, namely ilmenite, rutile, and anatase. read more, Organic chemistry is somewhere between inorganic chemistry and biochemistry. DOI: 10.3109/15563651003627777, Division of Research Safety
It has a molar mass of 35.04 g/mol. When handling large amounts (>500 mL), or when splashing is likely to occur, wear additional protection to prevent skin contact, especially when using TMAH: A face shield above splash goggles, base-resistant gloves with long cuffs, and a base-resistant apron or smock. as a neutralizing chemical, we generally recommend against its use. 2019 Copyright Digital Analysis Corporation. This means that it can form a completely homogeneous mixture with water. .jpg) At last, with some important points of this article on Is NH4OH an acid or base? Ammonium hydroxide itself is an aqueous solution of ammonia. Close the door and keep people from entering. Ammonium hydroxide is a solution of ammonia in water that appears as a colorless liquid with a highly pungent odor having a pH value between 7 to 10. A 1 Normal (Molar) solution of Ammonium Hydroxide contains approximately 68 mL of concentrated Ammonium Hydroxide per liter. The concentration of ammonia in ammonium hydroxide solution, which can be up to 30%, makes it toxic. These bases It is used as a cleansing agent in many household works. Have a spill kit readily available before working with concentrated bases. Move into fresh air immediately. Lets check whether NH4OH fulfills the requirement for classifying as Bronsted-Lowry base or not. Seek medical attention. Lets understand why NH4OH acts as the weak base with the help of the dissociation constant value concept. However, this small amount of product is able to reduce the pH appreciably; in this case from 7.00 to 3.38. But the the favor of reaction mostly lies to the left side that means large number of NH3 will present in the aqueous solution as compare to its right sided product(NH4+ and OH).
At last, with some important points of this article on Is NH4OH an acid or base? Ammonium hydroxide itself is an aqueous solution of ammonia. Close the door and keep people from entering. Ammonium hydroxide is a solution of ammonia in water that appears as a colorless liquid with a highly pungent odor having a pH value between 7 to 10. A 1 Normal (Molar) solution of Ammonium Hydroxide contains approximately 68 mL of concentrated Ammonium Hydroxide per liter. The concentration of ammonia in ammonium hydroxide solution, which can be up to 30%, makes it toxic. These bases It is used as a cleansing agent in many household works. Have a spill kit readily available before working with concentrated bases. Move into fresh air immediately. Lets check whether NH4OH fulfills the requirement for classifying as Bronsted-Lowry base or not. Seek medical attention. Lets understand why NH4OH acts as the weak base with the help of the dissociation constant value concept. However, this small amount of product is able to reduce the pH appreciably; in this case from 7.00 to 3.38. But the the favor of reaction mostly lies to the left side that means large number of NH3 will present in the aqueous solution as compare to its right sided product(NH4+ and OH).